Which of the Following Is the Most Acidic
In the nuclear reaction one nuclide is. In m-chlorophenol electron withdrawing group CI is present at meta position.
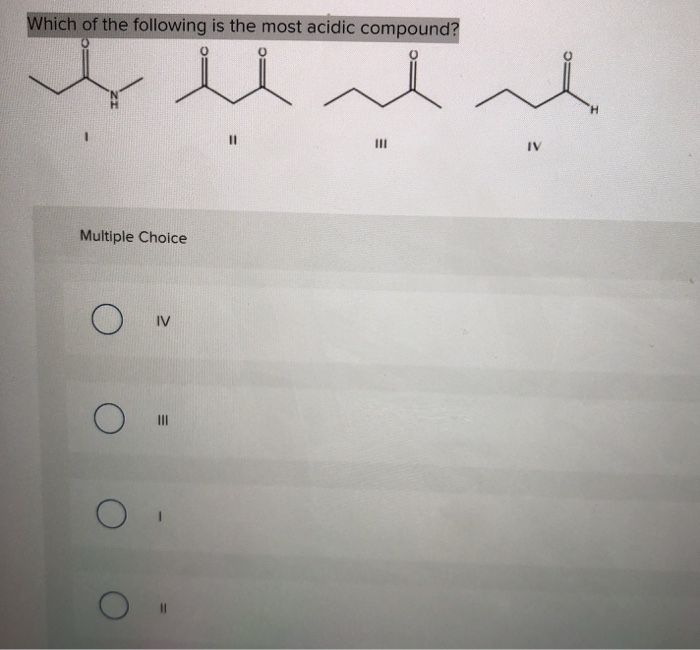
Solved Which Of The Following Is The Most Acidic Compound Chegg Com
These maetallic oxides powerful bases while Al2O3 is metallic oxide of p block which has comapratively more acidic character.
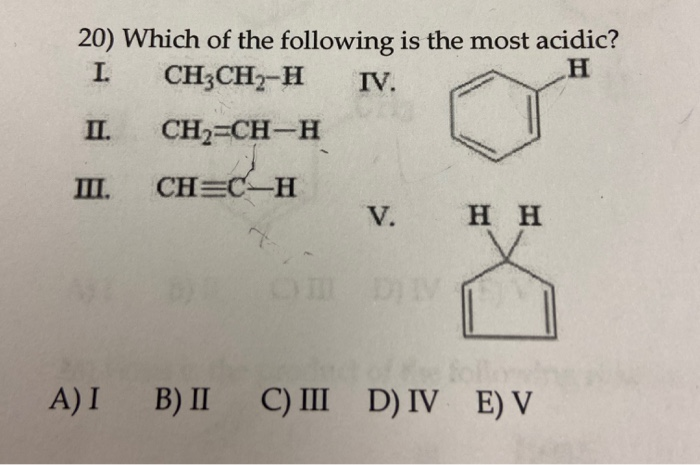
. A solution with a pH 14 b. Which of the following is the most acidic. So compound B is most acidic.
Solve any question of The p-Block Elements with-. Which of the following is the most acidic1 HCOOH2 CH3COOH3 CH3CH2COOH4 CH3CH2CH2COOH Get the answers you need now. Folyo OnH is the most acidic Presence of FC - group weakens the - 0-H bond there by facilates the the release of H ion easily.
Which of the following is the most acidic. Therefore the correct answer is D. A solution with pH 66 a solution with pOH 98 a solution with a hydroxide ion concentration -90 10-4 M a solution with a hydronium ion concentration - 90 x 10 M Select the correct answer below.
The given reaction is a nuclear reaction. 55 L of carbon dioxide gas in kiloliters e. So m-chlorophenol is most acidic among all the given compounds.
This is because resonance effect is stronger than the Inductive effect which operates in p-nitro benzoic acid. State the following measured quantities in the units indicated. Hence it is the most acidic oxide among all.
Another reason of acidic strength of N 2 O 5 is that the electronegativity of N is maximum in the given Vth group elements. The simplest way to understand is that the withdrawal of electrons leads to increase in acidity whereas donation of electrons decreases it. Which of the following is the most acidic.
Xon с с D Hot. More the delocalization more will be the stability so the anion of compound B is more stable than anion of compound D. Na2O MgO CaO are metallic oxide of s block.
35 mm in centimeters f. Which of the following is most acidic. From the above table its is clear that p nitro benzoic acid is the most acidic in nature.
So the most acidic is HCOOH. Correct option is C Na2O MgO CaO are metallic oxide of s block. Solved Answer of MCQ Which of the following solutions is the most acidic.
Please log in or register to add a comment. Rubidium-96 cesum-135 X --- uranium-235 A. A solution with a pH 12 c.
- a Blood plasma - b Gastric juice - c A solution with pH 7 - d A solution with hydronium ions at a concentration of 105 molL - Chemistry for Physiology Multiple Choice Question- MCQtimes. Presence of electron withdrawing group increases the acidic strength. Chemical Reactions of Alcohols and Phenols - Reactions Involving Cleavage of O-H Bond.
Which of the following solutions is the most acidic. 209 nm in millimeters h. A solution with a pH 7 d.
A solution with pH 66 w solution with pOH - 98 a solution with a hydroxide ion concentration a solution with a. I Benzyl alcohol ii Cyclohexanol iii Phenol iv m-Chlorophenol. These maetallic oxides powerful bases while Al2O3 is metallic oxide of p block which has comapratively more acidic character.
2500 mL of hydrochloric acid in liters c. As we know that on increasing the electronegativity character acidic nature increases. So the correct answer is Option A.
Find an answer to your question Among the following which solution is the most acidic. A solution with a pH 13 e. C H 2 C H C H 3 C H 2 C H - C H 3.
A 1-butyne b 2-butyne c 1-butene d Butane 56. 500 000 μg in kilograms. OH- 1 10-7 molL pH 10 H 1 10-5 m samuelmosesdavid samuelmosesdavid.
The acids form anion so we always check the stability of anion to determine the acidity. - Sarthaks eConnect Largest Online Education Community. 05 g of sodium in kilograms d.
Advertisement Remove all ads. Hence it is the most acidic oxide among all. Asked Apr 20 in Chemistry by Sowaiba 712k points Which one of the following is the most acidic.
The conjugate acid of CH3 CH₂ NH₃ CH₃ CH₂ Ä H₂ is - a 7 The acids that can are- protonale CH₃ Utzo H₂SO4 F. All of the solutions are basic. Which of the following resonance structures contributes the.
Complete and balance the following nuclear equation. 8740 m in kilometers g. So compound B will lose protons more easily than compound D.
Is thus the most acidic in nature. Which of the following is most acidic. ClCH 2 CH 2 OH.
Alpha carbon of benzyl alcohol and cyclohexanol is sp3hybridised while in phenol and m-chlorophenol it is sp2hybridised. This happens because the electron-withdrawing groups delocalize the charge of the carboxylate ion making it more acidic. 150 mg of aspirin in grams b.
So N 2 O 5 is most acidic.
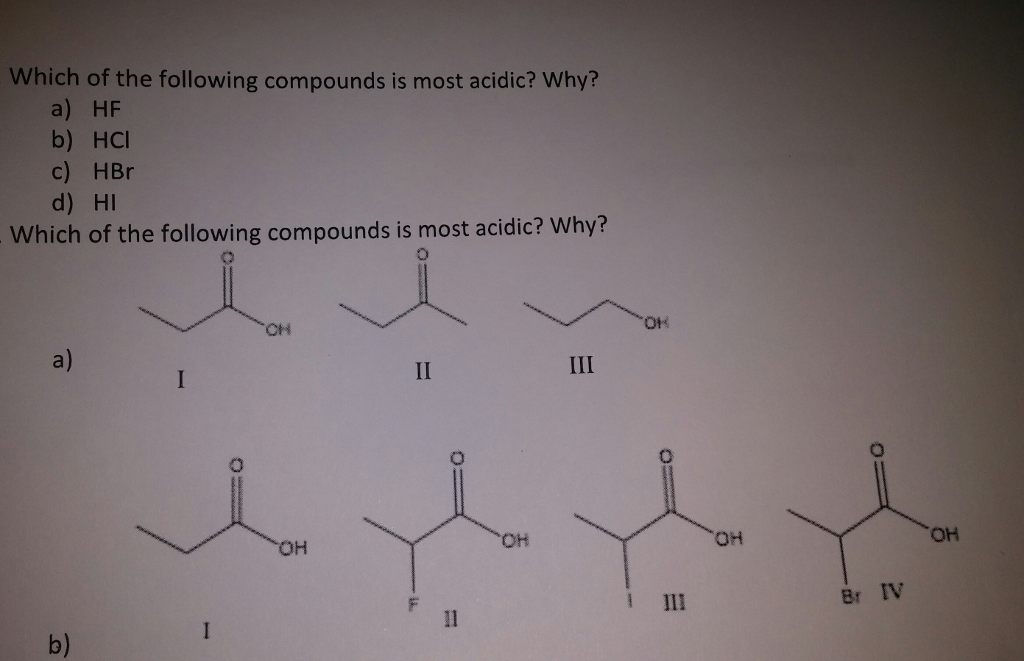
Solved Which Of The Following Compounds Is Most Acidic Why Chegg Com

Which Of The Following Is The Most Acidic In Nature Which Of Theconsider Attachment Brainly In
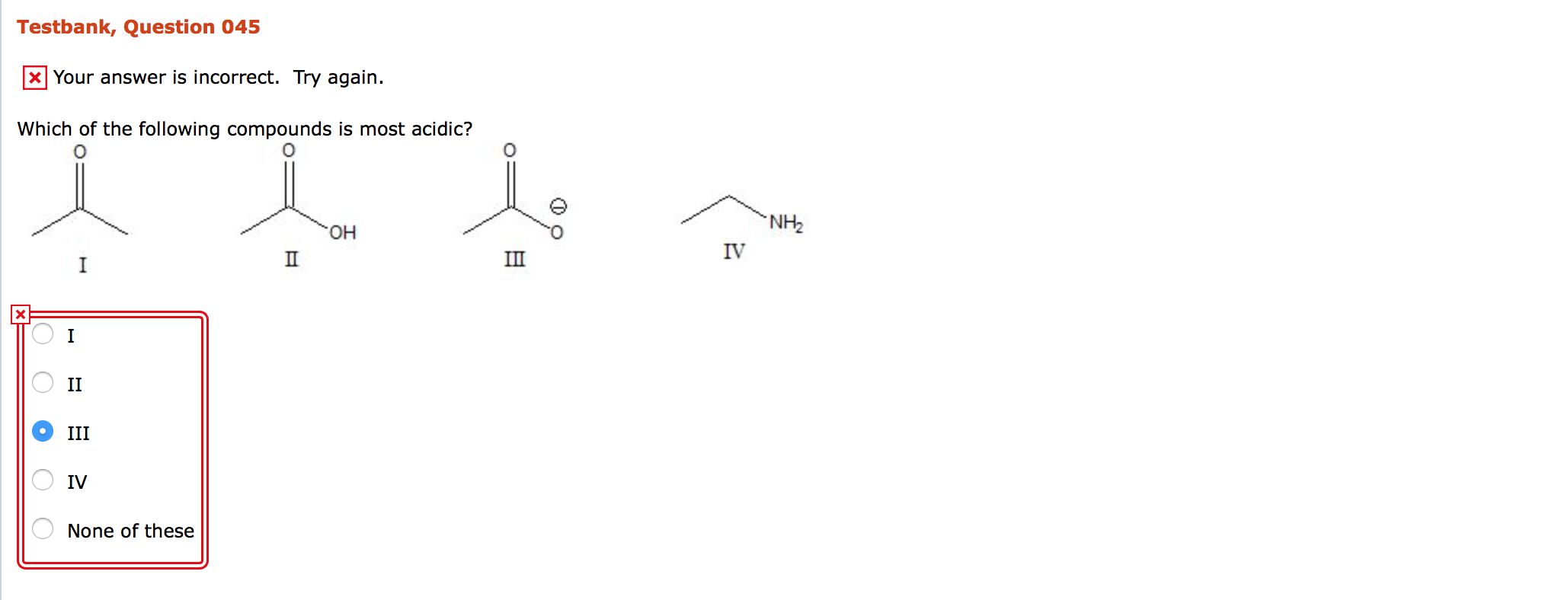
Solved Which Of The Following Compounds Is Most Acidic I Chegg Com

Solved 20 Which Of The Following Is The Most Acidic I Chegg Com
No comments for "Which of the Following Is the Most Acidic"
Post a Comment